1. Problèmes connus
Sonde Dragonfly
- Risque de formation de givre sur la vitre et dans les boutons (sera résolu en v2.1)
- Les températures lues par la sonde ne sont pas précises (sera résolu avec la pré-certification en v2.1)
- Le prototype actuel est sensible aux perturbations électromagnétiques, mais dans ce cas il redémarre et continue dans le même état que précédemment
Application Fleet Manager
- Navigation dans la tracking map (carte) et manipulation du graphiques des températures peu ergonomiques
- Sur les appareils mobiles, le fleet manager peut arrêter de se rafraichir automatiquement
2. Démo
- Suivre la livraison du Dragonfly
- Sortir le Dragonfly et attendre qu’il se réchauffe pour provoquer la sortie de règle opérationnelle
- Vérifier les mises à jour du status dans Fleet Manager
- Passer le Dragonfly en returning en appuyant 2 secondes bouton du bas (►)
- Valider le returning dans Fleet Manager
- Remettre le Dragonfly dans boîte et renvoyer à ESNAH
Merci de répondre aux KPI (télécharger) et de les envoyer à
3. Prochaines étapes
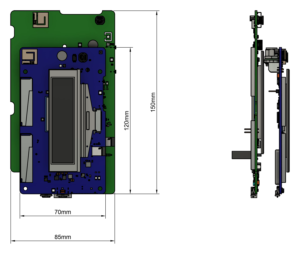
- Nouveau PCB de taille réduite
-
Amélioration et calibration de la sonde thermique
-
Amélioration de l’autonomie de la batterie
- Désunion automatique des trackables et trackers suivant configuration
- Certification
- Amélioration du blindage pour une meilleure immunité aux perturbations électromagnétiques
- Renforcement de la sécurité et du stockage des données
- Application mobile compagnon pour configurer le Dragonfly
- Capteur x-Ray (en beta)