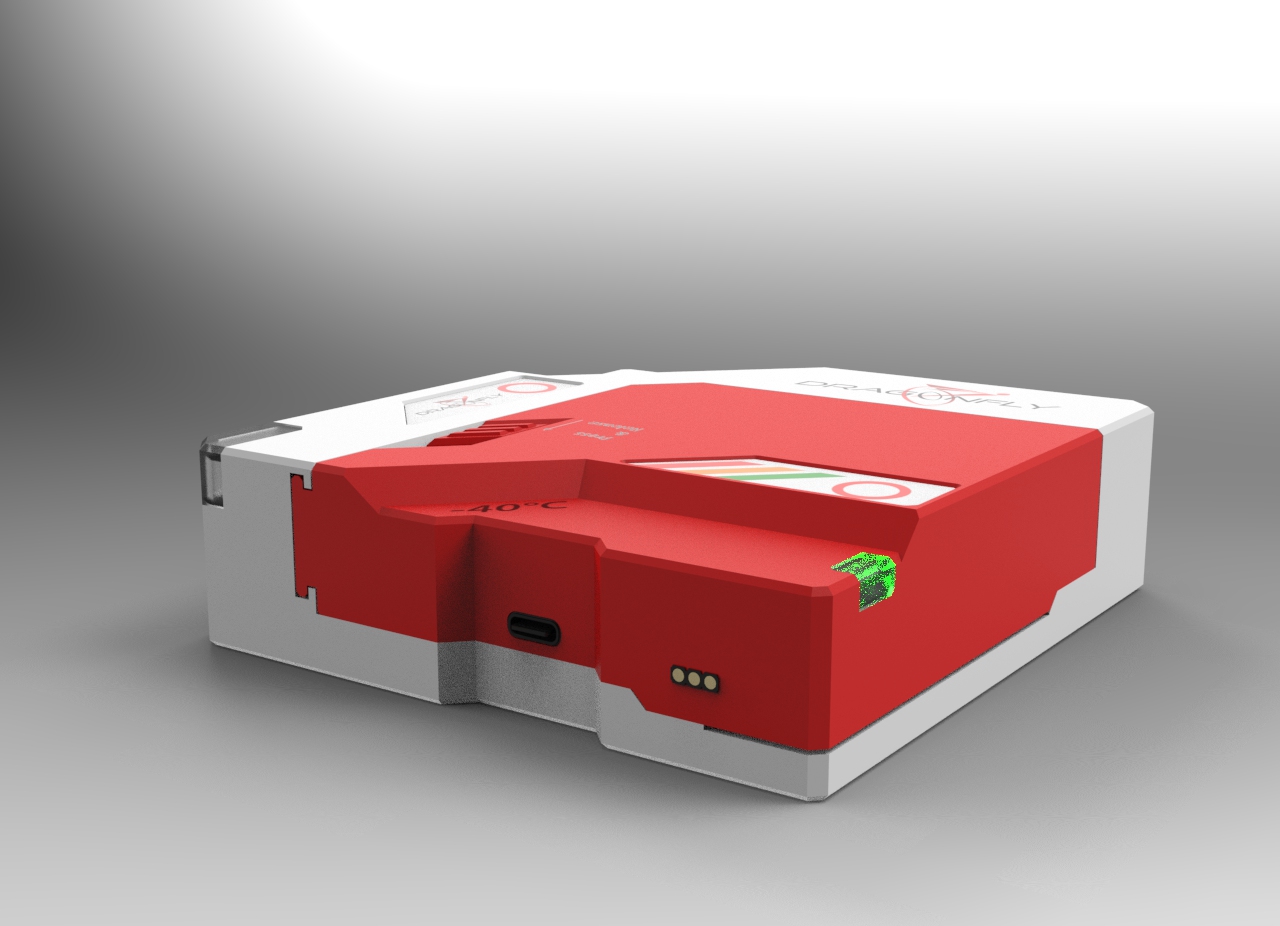
The objectives
DragonFly is a stand-alone certified solution (hardware, firmware and software) for Covid Vaccine monitoring, tracking and geolocation by air, marine and land.
The device is fully autonomous, with machine learning and offer a real time warning/alert notification service. DragonFly is expected to improve safety, coordination and information exchange of the pharmaceutical lot, through all the players of the supply chain. DragonFly will avoid counterfeit, and provide a lifecycle management during the shipping of drugs.
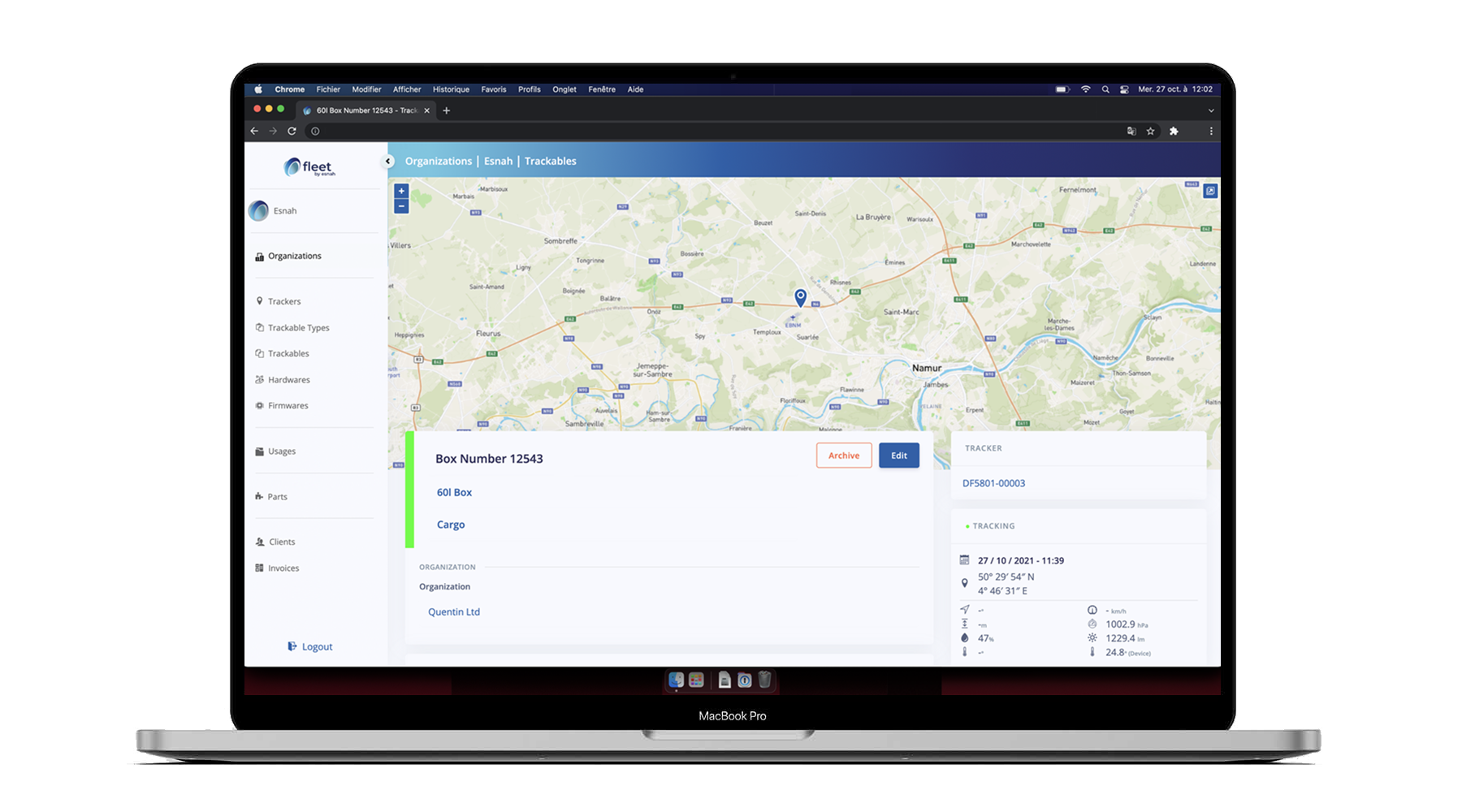

The solution addresses the entire logistic chain for medicine transportation:
- The Pharmaceutical firm wants to have traceability certification.
- The Logistic company has to meet the traceability requirements.
- The Hospital needs to be sure that the products are in good quality.
Concept
The goal is to monitor pharmaceutical packaging with near real-time information on the inner environmental condition of the vaccine. As the DragonFly is linked with the pharmaceutical lot, it contains the operational rule for the transport (temperature threshold, acceleration and vibration pattern, authorized opening zone, …) in order set the transport condition for an end-to-end lifecycle management.
The information generated by embedded sensors (environmental (temperature, humidity, UV, shock, …) / inspection / location) will avoid counterfeit, support decisive decision and actions towards the safety of the pharmaceutical product and will certify the transport conditions.